2021 Covid Vaccine Comparison Guide
Jan 29, 2021
The United States has two COVID-19 vaccine types that have been shown to be highly effective in clinical trials and have been approved for emergency use authorization. The vaccines were developed by Pfizer-BioNTech and by Moderna. Both of these vaccine companies were part of Operation Warp Speed, a partnership among components of the Department of Health and Human Services (HHS), including the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), the Biomedical Advanced Research and Development Authority (BARDA), and the Department of Defense (DoD). The goal of Operation Warp Speed is to produce and deliver 300 million doses of safe and effective COVID-19 vaccines with the initial doses available in January 2021.
Both the Pfizer Vaccine and Moderna Vaccine have shown essentially equivalent degrees of efficacy to reduce the risk of severe Covid disease. Despite similar production technologies, Pfizer and Moderna’s vaccines have differences such efficacy, storage protocols, and availability. Both vaccines use mRNA technology to trigger an immune response instead of putting a weakened or inactivated germ into the body. The mRNA COVID-19 vaccines teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies. That immune response, which produces antibodies, is what protects us from getting infected if the real virus enters our bodies. The Johnson & Johnson vaccine has a slightly lower efficacy of 66%, however, it only requires a single dose and is easier to store. Unlike the Pfizer and Moderna vaccines, the Johnson & Johnson vaccine uses double-stranded DNA.
While the vaccine supply will be limited at first, populations that have been disproportionately affected by COVID-19 will be prioritized in the Kansas Department of Health & Environment (KDHE) phased plan. As the supply increases, vaccines will become available to all Kansans — regardless of ability to pay — and will be distributed at convenient locations across the state. HealthCore Clinic will be an official COVID-19 vaccine site in Wichita, Kansas.
Vaccine Companies
While there are many companies that have either been FDA approved or are still in the pharmaceutical study process, the Pfizer-BioNTech and Moderna vaccine types were the first to be approved in America. Here are the top COVID-19 vaccine companies in the United States:
Pfizer-BioNTech was authorized December 11, 2020 by the U.S. Food and Drug Administration (FDA) and began to be administered to people in high-risk groups in the United States, including health care workers and people living in nursing homes. On December 18, 2020 the FDA authorized Moderna’s vaccine for emergency use in people 18 years or older after successful clinical trials. On February 27, 2021, the FDA authorized the third vaccine for the prevention of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The authorization allows the Janssen COVID-19 Vaccine (Johnson & Johnson vaccine) to be distributed in the U.S. for use in individuals 18 years of age and older.
Covid Vaccine Comparisons
Each of the COVID-19 vaccine types available to Kansans has subtle differences that affect how they are produced, stored, and administered. The main difference is efficacy and doses.
Pfizer-BioNTech
The Pfizer-BioNTech vaccine has an efficacy of 95% and requires 2 shots, 3 weeks apart. It is an mRNA-based vaccine, which triggers an immune response that produces antibodies to protect us from getting infected if the real virus enters our bodies.
- 95% efficacy
- 2 shots, 3 weeks apart
- mRNA-based
Moderna
The Moderna vaccine has an efficacy of 94.5% and requires 2 shots, 4 weeks apart. It is also an mRNA-based vaccine, which triggers an immune response that produces antibodies to protect us from getting infected if the real virus enters our bodies.
- 94.1% efficacy
- 2 shots, 4 weeks apart
- mRNA-based
Johnson & Johnson
The Johnson & Johnson vaccine (Janssen COVID-19 Vaccine) has an efficacy of 66% and only requires a single dose. This vaccine is based on the COVID-19 virus’s genetic instructions for building what’s called a “spike protein.” Unlike the Pfizer and Moderna vaccines, which store the instructions in single-stranded RNA, the Johnson & Johnson vaccine uses double-stranded DNA.
- 66% efficacy
- 1 shot
- DNA-based
Vaccine Dosage Details
The COVID-19 vaccine is currently available in very limited doses, so planning efforts by the KDHE are based on phases of availability. As availability increases, vaccines will be opened up to all Kansans and distributed at convenient locations such as HealthCore Clinic in Wichita. Kansans cannot be denied a vaccine if they cannot afford the administration fee or do not have insurance.
The Pfizer and Moderna Covid vaccines require two doses to offer the full benefit. The first dose helps the immune system create a response against SARS-CoV-2, the virus that causes COVID-19. The second dose further boosts the immune response to ensure long-term protection. The Johnson & Johnson Covid vaccine only requires a single dose.
When you get vaccinated, you will receive a vaccination card or printout that tells you what COVID-19 vaccine you received, the date you received it, and where you received it. If you received the Pfizer or Moderna vaccine, you will require a second dose roughly 3-4 weeks (28 days) later.
Covid Vaccine Side Effects
Each vaccine type can induce similar side effects after being injected into the body. The most commonly reported side effects, which typically lasted several days, were pain at the injection site, tiredness, headache, muscle pain, chills, joint pain, swollen lymph nodes in the same arm as the injection, nausea, and vomiting, and fever. More people experienced these side effects after the second dose than after the first dose. These types of side effects are not unusual with vaccines and is a sign that your body is developing an immune response.

Some instances of allergic reactions to the vaccine have been reported. If you have had an immediate allergic reaction—even if the reaction was not severe—to a vaccine or injectable therapy for another disease, ask your doctor if you should get a COVID-19 vaccine. Your doctor will help you decide if it is safe for you to get the COVID-19 vaccine.
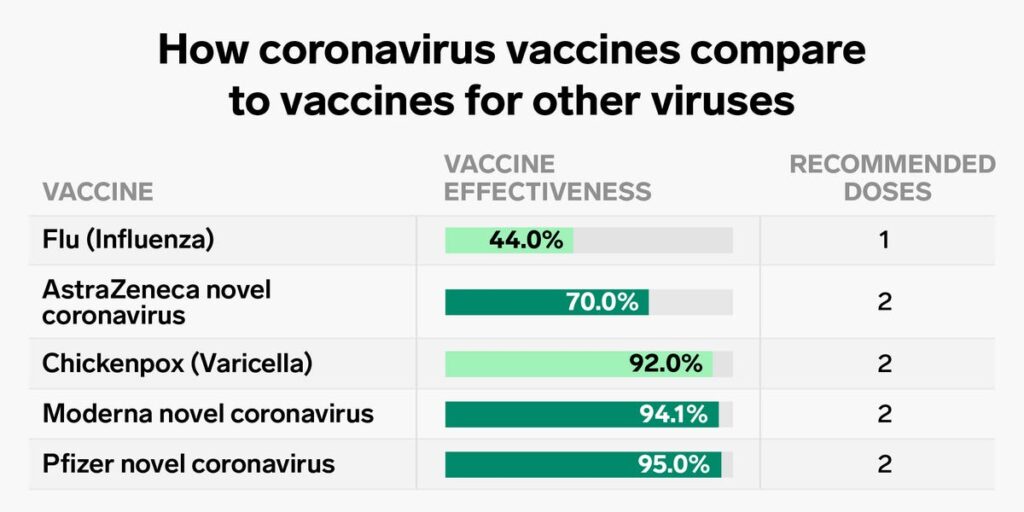
Vaccine Efficacy
Efficacy refers to the ability of the COVID-19 vaccine to successfully produce antibodies to protect recipients from getting infected if the real virus enters their bodies. Both the Pfizer-BioNTech and Moderna vaccine types have similar efficacy — Pfizer-BioNTech has 95% efficacy and Moderna has 94.1% efficacy. The AstraZeneca vaccine has an efficacy of 70%.
Production Capacity and Doses
The goal of Operation Warp Speed is to produce and deliver 300 million doses of safe and effective COVID-19 vaccines with the initial doses available in January 2021. The U.S. is on track to secure 100 million doses of Moderna’s vaccine by the end of March 2021 and an additional 100 million by June 2021. Pfizer-BioNTech expects to deliver 200 million doses by July 31, 2021. Because two doses are required per person, these 400 million combined doses would allow for 200 million people to be vaccinated in the United States.
The federal government provides the vaccines to the states. The State of Kansas, through KDHE, then distributes the doses to vaccine sites such as HealthCore Clinic in Wichita to administer to the general population.
Coronavirus Vaccine FAQs
How does the Coronavirus Vaccine work?
Both the Pfizer and Moderna vaccines use messenger RNA (mRNA) to encourage your cells to initiate an immune response to COVID-19. Our body relies on proteins every day to keep us healthy. Our body uses mRNA to tell our cells which proteins to make – antibodies in this case.
How will we know if the vaccines work?
Clinical trials provided data and information about how well the Covid vaccine types triggered an immune system response against SARS-CoV-2, the virus that causes COVID-19. The duration of immunity is currently unknown.
Are antibodies the only form of immune protection?
Yes. Covid vaccines help the immune system create a response against SARS-CoV-2, the virus that causes COVID-19. You should still get vaccinated even if you’ve had COVID-19 because we are seeing evidence of reinfection.
Does the Covid vaccine stop you from getting Covid?
It takes a few weeks for the body to build immunity after vaccination. It is possible a person could be infected with the virus that causes COVID-19 just before or just after vaccination and still get sick. This is because the vaccine has not had enough time to provide protection.
If you get the COVID 19 vaccine will you still need to wear a mask in public?
Yes. Medical experts need to understand more about the protection that COVID-19 vaccines provide in real-world conditions before making that decision. We don’t yet know whether getting a COVID-19 vaccine will prevent you from spreading the virus that causes COVID-19 to other people, even if you don’t get sick yourself.
Can I get COVID-19 from the vaccine?
No, you cannot get COVID-19 from the vaccine.
Most people recover. Why do I need a vaccine?
There is no guarantee of recovery and the long-term effects on people who have had the virus is unknown. The vaccine, like other vaccines, has the greatest ability to stop the spread of the virus.
When will I be a COVID-19 vaccine candidate?
There are five phases in the Kansas vaccination process. To find out what phase you are a vaccine candidate for, visit the Kansas Vaccine Website. You can also sign up to learn more and receive a notification from HealthCore Clinic when it is your turn on our COVID-19 Vaccine Info page.